Science is a place where – what you find in nature, pleases you. – Subramanian Chandrasekhar
The scientific community was not in total agreement with the atomic model and molecular energy during 1800. Many breakthroughs in the research were in the coffer. We were then at the doorstep of modern physics.
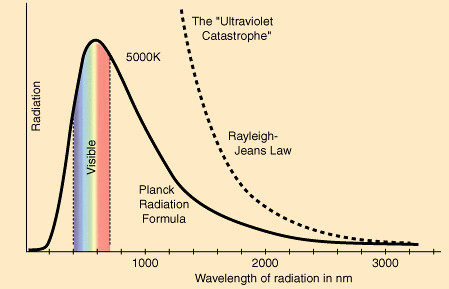
The light was always perceived as energy long before modern science, but Einstein scientifically proved it. Before Einstein realized light as a packet of energy, Max Planck and Einstein were baffled with the ultraviolet catastrophe in analyzing black body radiation. So, we will focus on this subject as it is the doorway to quantum physics. What is an ultraviolet catastrophe? A blackbody is a theoretical object which absorbs all radiation falling on it; it is also a perfect emitter of radiation over all wavelengths. We are now unconsciously thinking energy as a waveform, but not its discrete nature.
Classical physicists knew atom and atomic energy but they were trying to model it around spring energy with infinite and continuous states. As classical physics got quite mature with more understanding of thermodynamics, debates were resolved with knowledge of atom, molecule & energy relationship of atomic structure. Boltzmann and other scientists realized from the relationship of pressure, volume, and temperature that each atom has a unique energy and guided by a universal constant (Gas constant and eventual Boltzmann constant) with energy and temperature relationship. Mathematically and experimentally scientists started to calculate the average energy in an atom. Thus the art of quantization started.
The energy in a container with N number of an atom in the gas molecules can be written as PV=NkT; k =1.4 times 10-23 Joules/Kelvin; Energy per atom is kT, where P is pressure, V is the volume, T is temperature, and k is Boltzmann constant. Due to the randomness of contained atoms’ energy, scientists measured the distribution of energy for atoms, where kT is the average energy per atom.
A few years later from the photoelectric effect Einstein found light is a packet of energy named photon. Gradually, many ideas over time unfurled the knowledge to quantum physics partially. So we should understand these steps before we jump into quantum physics. We have heard ultraviolet catastrophe in black body radiation and Plank constant as articulated by Max plank (23 April 1858 – 4 October 1947). Incidentally, Plank was also a gifted pianist. One of his sons was executed for an unsuccessful attempt to assassinate Hitler in 1944.
About the same time of Plank, Rayleigh (12 November 1842 – 13 June 1919) and James Jeans (11 September 1877 – 16 September 1946) worked on a spectrum of blackbody radiation with a new idea called Equipartition theorem (classical statistical physics) and explained that the total kinetic energy of a system is shared equally among all of its constituents once the system reached thermal equilibrium. Rayleigh-Jeans Law derived the spectral radiance of electromagnetic radiation as a function of wavelength from a black body at a given temperature through classical arguments. They came up with a law called Rayleigh Jean curve. It is mathematically given as “B = V2kT/c2”. Where B is the radiant energy, there is no need to understand the equation for now. Unfortunately, the assumption of the Infinity energy state in classical physics caused the failure in the calculation for high frequency against experimental radiation. This failure of classical physics is known as the ultraviolet catastrophe. Rayleigh curve shows radiation energy asymptotically increasing from the UV region and higher frequency.
It had been resolved in 1900 with the derivation by Max Planck (April 23, 1858 – October 4, 1947) of Planck’s law, which gave the correct radiation at all frequencies.
All objects with a temperature above absolute zero degrees Kelvin emit energy in the form of electromagnetic radiation. The spectral distribution of the thermal energy radiated by a blackbody depends only on its temperature.
Courtesy of Google
Plank hypothesized atomic vibrations were not continuous but discrete; Planck theorized that a minimum amount of energy is needed for atomic vibration. It is analogous to minimum torque needed to unscrew a bottle, you must have experienced this effect. He stated that minimum energy should be equivalent to the frequency of vibration of atom multiply with a very small constant number, that number is known as Plank Constant.
This minimum energy E, that was proportional to the frequency of its associated electromagnetic wave. He was able to calculate the proportionality constant, h, from the experimental measurements,
Planck decided to assume that atoms could only vibrate at certain frequencies that were whole number multiples of some base frequency, which he called h. In other words, atoms could vibrate at the h frequency, or 2h, or 3h, but not 2.5h. The quantizing concept is now embedded in modern physics. The tiniest energy is quantized. This assumption fits the blackbody radiation for all frequencies.
Further study by Einstein and modern scientists indicated that Planck’s physical constant is the quantum of electromagnetic energy carried by a photon. Modern physics can’t be explained without eponyms h (plank constant). All the modern principles need the use of plank constant for their mathematical derivation.
To name a few of these principles are Heisenberg uncertainty principle, De Broglie wavelength, Schrodinger equation, Energy levels of electron orbits, the relation between energy and frequency of a photon. Utilizing the use of modern measurement techniques scientists’ community agreed with Plank constant value 6.63 times 10-34 Joule second. Please continue the next few chapters before entering the world of quantum physics.
















Comments »
No comments yet.
RSS feed for comments on this post. TrackBack URL
Leave a comment