“The important thing is not to stop questioning. Curiosity has its own reason for existing” as Albert Einstein said. All my writings are out of curiosity. I am trying my best to maintain the flow so that it maintains some sense of continuity from the previous publications. Please refer to previous publications.
CV Raman
Chandrasekhara Venkata Raman was born on 7th November 1888 in India. His contribution to the field of light is enormous. It gave quantum physicists many tools for the study of the universe. He used to say “if you ask the right questions, nature will open the doors to her secrets.” His contribution to light and spectroscopic idea are notable.
He married to Lokasundari at a very early age; the couple went to Calcutta for a job. He taught at Calcutta University. They lived in a flood porn zone of Calcutta, once the street in Calcutta was flooded and Raman was very mad and loud with the situation and feeling helpless to go work; seeing him at a loss, his wife quickly took two stools and helped Raman to walk through the waterlogged street, while she was changing the position of the stools alternatively and wading through the water until he reached high ground. A simple example says everything about Lokasundari. In his later years, Raman started doing gardening especially with flowers and jokingly claiming that he deserves second Nobel Prize. His wife had the perfect answer. She replied, “With one Nobel you were intolerable, with two you would be insufferable.”
Spectroscopic Notation
From the ancient time human being were fond of light, darkness only gave them fear. A great many scientists tried to analyze light to its smallest constituent (Photon), they watched the burning wood emits a different kind of light, they questioned WHY? This branch of physics gave us knowledge of spectra of atoms and assigned letters to the characteristic spectra. Atomic scientists started to use these letters to note electron energy and its states and use quantum number designations of electron states, the notation is as follows:
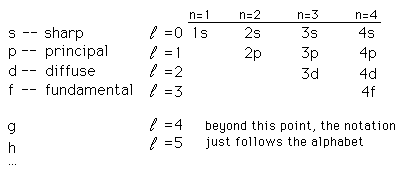
Courtesy of Google
How orbitals are filled
Stability is the ultimate goal of nature; it is true for large objects and smallest particle like an atom. Nature had created all elements with this principle. As electron orbits around the nucleus, the lowest energy level stays closer to the nucleus. Highest energy levels stay farther and further away from the nucleus in accordance with ascending energy level. Thus nature has created many different stable elements which stay happily in their ground state until external force disturbs the binding energy of electrons. Disturbance makes an electron to jump from one orbit to another orbit. In some case, it will emit energy and in other cases, it will add the energy to the element.
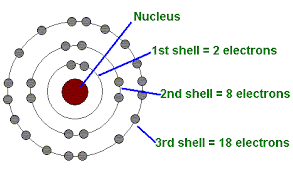
The 1st energy level is closest to the nucleus
Contain sublevels (Four types); notations are used as follows:
s (lowest energy)
p
d
f (highest energy)
| Energy Level | Sub level | Total number of electrons |
| 1 | s | 2 |
| 2 | s,p | 8 = 2+6 |
| 3 | s,p,d | 18 = 2+6+10 |
| 4 | s,p,d,f | 32 = 2+6+10+14 |
| 5 | s,p,d,f | 32 |
| 6 | s,p,d,f | 32 |
| 7 | s,p,d,f | 32 |
The 2nd level of energy, p sub level is denoted by 2p, 3rd level of energy d sublevel is denoted by 3d, and so on.
3rd energy level d sublevel > 4th energy level s sublevel
So electrons will fill a 4s sublevel before it fills a 3d sublevel
Each sublevel contains at least one orbital. Example energy level 2 can have a maximum of 4 orbital.
Area of the higher probability of finding electrons
Every orbital holds 2 electrons
Always fill the lowest energy levels and sublevels first.
Typical Notations
Courtesy of Google
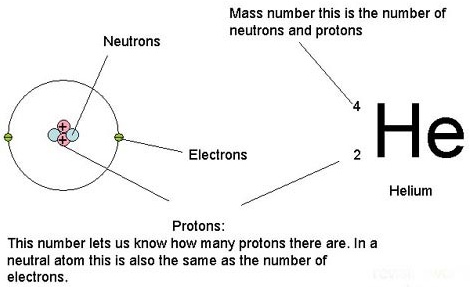
Atomic number / Atomic mass number:
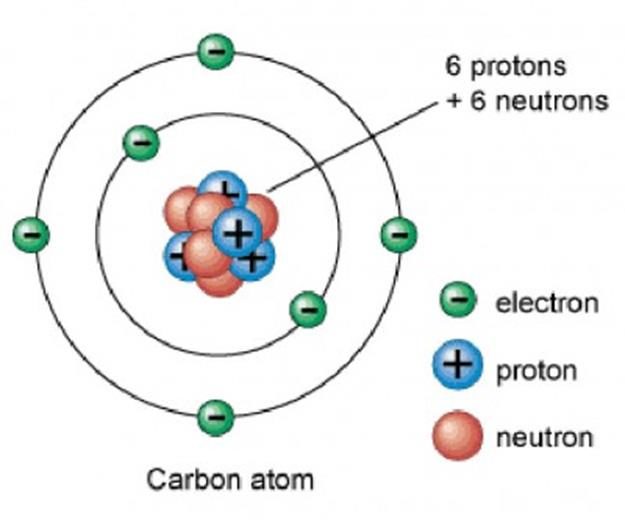
Let’s look at Carbon.
Courtesy of Google
The atomic number of Helium is 2 (number of protons)
Each atomic number identifies a specific element; Carbon has 6 protons so its atomic number is 6. The number of nucleons (both protons and neutrons) in the nucleus is the atom’s mass number.
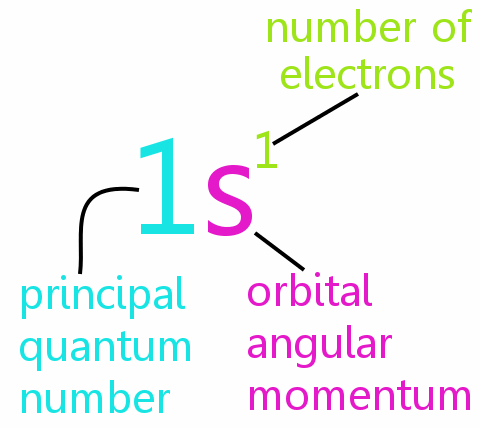
The notation used to describe an atom for elements as follows:
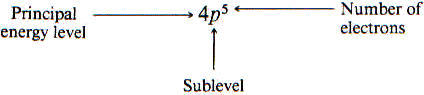
Electron Configuration – shorthand notation for showing what sublevels are filled-
- Carbon atom has 6 electrons – 1s2 2s2 2p2 because 2+2+2=6
- Silicon atom has 14 electrons – 1s2 2s2 2p6 3s2 3p2 because 2+2+6+2+2=14
By nature, electrons are filled according to lowest energy sublevels first.
Understanding of energy is the most important of all. Since the big bang and thereafter all kind of material are created. Scientists understood how atom worked, then how element formed out of those atoms. So we will look into the atomic structure in great detail. This will give us a better understanding of we have evolved. Again, we will start by saying there are four forces as scientists defined how elements are dependent on the forces.
The “Pauli exclusion principle” constrains the entire buildup process of elements.
It states that only one electron to occupy each available state. Remember nature’s final goal is to stay in a stable state or to the lowest energy state; each individual states will fill in the order of ascending energy.
Let us picture the Periodic table for few elements. We will try to understand the technique is used by nature to make the first few elements. We must not forget that billions of years ago hydrogen was in abundance and nature slowly gave birth to other elements.
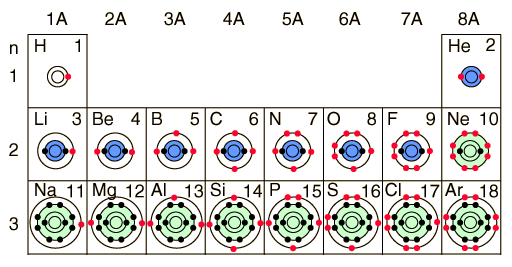
Courtesy of Google
Electron spin is an intrinsic property of electrons. Electrons have intrinsic angular momentum characterized by quantum number s = 1/2.
The 1s spatial state can have two states of electron spin, which we usually just refer to as “spin up” and “spin down”. With two electrons in the 1s state, it is filled and stable, forming the noble gas helium. It is chemically unreactive.
Lithium has only one electron in the second shell. It has a tendency to lose that outer electron and change to the stable helium electron structure.
Following pictorial view will give us some insight into the building process. Atom has a finite dimension.
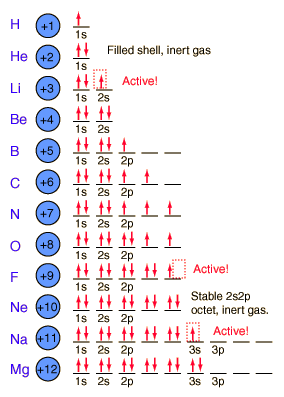
Courtesy of Google
The elements from boron to neon in the periodic table are filling the 2p orbital. The three possibilities for 2p orbitals can be associated with spatial directions. The order of filling will place one electron in each before placing two in any one orbital.
The filling of the 2p orbital places eight electrons in the second shell, a “stable octet”. The particularly stable configuration produces the noble gas neon.
But fluorine with one less than a stable octet is very active! It tends to gain one electron while the very active sodium tends to lose one electron, in each case producing the stable octet configuration of neon.
















Comments »
No comments yet.
RSS feed for comments on this post. TrackBack URL
Leave a comment